NV Logistics is proud to contribute to the restart of Archery competition !
Behind the scenes series #1
NV Logistics is proud to contribute to the restart of Archery competition !
Behind the scenes series #1
Biopolymers are chain-like molecules.
The image above to the left depicts the structure of the cellulose biopolymer.
This client researches, develops, manufactures and commercialises biopolymer-based medical devices.

“Aptissen” stands for Advanced Polymer for TISSue ENgineering, which unveils the company’s skills. These skills have been built over an 11 years period within the Healthcare division of the Swiss company Anteis SA.
The acquisition of Anteis SA (innovative injectable medical devices) by Merz Pharma (November 2013) gave Anteis’ founders the opportunity to spin off its Healthcare division and found Aptissen SA.
Aptissen’s products relieve patients in the field of Ophthalmology, with surgical solutions for glaucoma, and in the field of Orthopaedics, with innovative treatments for osteoarthritis.
A polymer (Greek poly-, “many” + -mer, “parts”) is a large molecule.
Biopolymers are elements that occur in nature.
Cellulose is the most common organic compound and biopolymer on Earth. About 33 percent of all plant matter is cellulose. The cellulose content of cotton is 90 percent, while wood’s is 50 percent.
Scientists and commercial entities are increasingly looking at biopolymers as an opportunity to commit to resource conservation, environmental preservation, and sustainable technologies.
Curious? Read more here:
Biopolymers for medical and pharmaceutical applications
.
.
For your easy reading, we have divided this report into three parts.
1. The Client’s Challenge |
|
2. NV Logistics’ Answer |
|
3. The End Result |
.
.
Back in July 2014, Aptissen was actively searching for a new warehouse facility to stock their merchandise at a guaranteed temperature of between 2° and 25° C.
Three well-defined imperatives dictated their search for a new storage supplier:
NV Logistics fitted the bill perfectly for all 3 imperatives and things moved forward swiftly: the commercial offer was submitted in July and signed only a few weeks later!
.
.
The contractual conclusion was straightforward because we were able to demonstrate high performance in all areas imperative to Aptissen. In addition, we earned the trust of Aptissen’s auditors from SGS whom we met on location and for whom we reserved quality time for expert discussions..
3 key points stand out and speak in our favour:
The moral of the story: busy clients appreciate a competent and certified supplier with a capacity for holistic services..
![]()
.
We believe that Aptissen’s needs for integrated services – which include certified storage facilities and ERP based processing systems – are fairly typical in the biotech, medical and pharmaceutical landscapes.
A year down the road, since July 2014, our relationship with Aptissen is a happy one. Here is what our client says: (Testimonial)
We are grateful to Aptissen who agreed to let us publish this Case Study, giving us the opportunity to unveil insights about our holistic, modern-day warehouse offering.
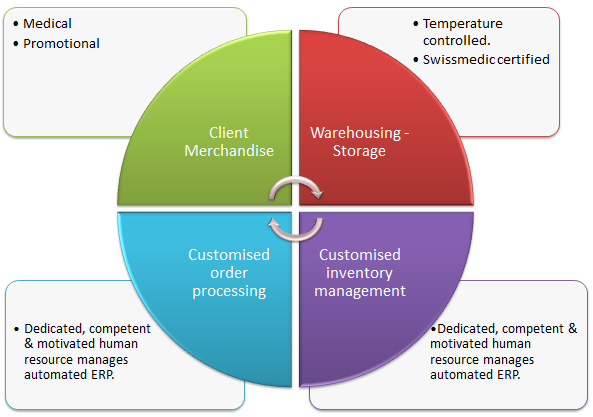
Aptissen needs storage for its medical devices and other diverse materials (packaging and marketing).
Required storage temperature for medical devices: +2° and +25° C.
NV Logistics stores Aptissen’s products at its facility at Geneva-Meyrin, Switzerland.
Facility details:
Invoice agreement with Aptissen: based on pallet units per month. Pallet size = 120x080x120 cm.
A close and trusting customer relationship is an essential component of our business approach. Upon signature of the contract, we immediately allocated a competent and motivated resource to the Aptissen account.
For the sake of this case study, let’s call him Mr. NV.
This person is fully trained to use NV Logistics’ customisable ERP* software.
NV Logistics owns the necessary technical resources to provide optimised processes, based on each client’s specific needs.
*ERP – Enterprise Resource Planning software
Want more details? Please read on!
Section 1 outlines the Order Processing flow, put in place for Aptissen.
Section 2 describes what happens with Inventory Management at ERP software level.
.
|
|
Mr. NV handles 3 key processes: |
| 1. Receives merchandise from Aptissen’s suppliers. | |
| 2. Fulfils orders and prepares merchandise to Aptissen’s clients. | |
| 3. Handles Client returns. |
Mr. NV takes action as follows:
We describe the ERP side of things in Section 2.
Mr. NV takes action as follows:
Based on Aptissen’s instructions, he prepares the order (turnaround = max. 1 working day).
We describe the ERP side of things in Section 2.
Mr. NV takes action as follows:
We describe the ERP side of things in Section 2.
.
Client Bonus: A detailed trace of a product’s inventory journey is available for online consultation by the client at all times.
|
|
Mr. NV handles 4 ERP software routines: |
| 1. Incoming (supplies) | |
| 2. Outgoing (order fulfilment). | |
| 3. Returns | |
| 4. Reporting |
.
Incoming products are immediately placed into the temperature-controlled Quarantine area and entered into the inventory system, labelled “Quarantine”.
ERP System process: the software creates a database record with a unique ID key which is completed by the manual entry of other identifying elements such as product reference, batch number, quantity, description.
Products in the Quarantine area are not available for “picking” (order fulfilment) before “release”.
“Release” is the approval given by Aptissen to move merchandise into the definite storage area, ready for order fulfilment.
ERP System process: once released, the database record is updated manually and the stock inventory is updated automatically.
Aptissen’s order fulfilment instructions include product reference, batch number, quantity, and the packaging person’s ID.
ERP System process: Only “released” merchandise is available for order fulfilment.
Only non-used merchandise is returned to NV Logistics.
Incoming products are placed into the temperature-controlled Quarantine area, awaiting Aptissen’s instructions:
a) release the product and move back into the definite storage area or
b) return the product.
ERP System process: if merchandise is once more released, the relevant database record is manually modified and the stock inventory updated automatically.
Bonus: Clients may punctually request targeted reports, providing them with useful statistical insights drawn from the ERP’s meticulously maintained and updated database.
.
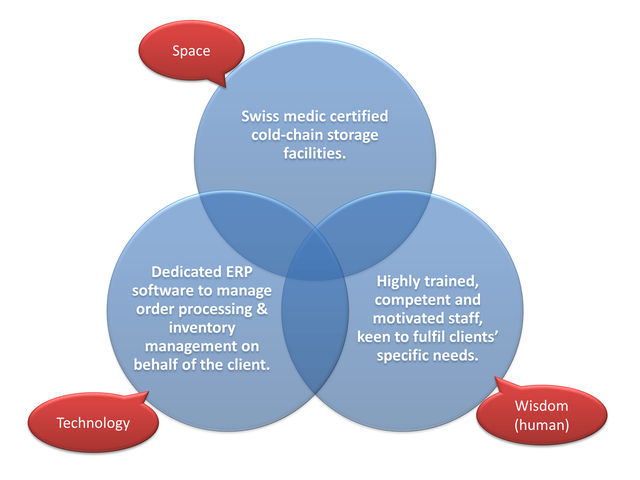
NV Logistics’ fully integrated services increasingly seduce and convince pharma, biotech and med tech companies in Switzerland.
The integrated, triangular approach brings together Swissmedic certified storage facilities, dedicated ERP software for customisable order processing & inventory management as well as highly trained, competent and motivated staff.
.Geneva University Hospital (HUG) is the largest medical establishment in Geneva city and canton.
It has a long history of excellence and is seen as one of the most important medical institutions in Switzerland.
Attached to the Faculty of Medicine of Geneva University, it is also a radiant research centre.
And what’s more, the hospital is an integral part of a region called West Switzerland, reputed as a fertile ground for innovation in biotechnology and the life sciences.
One of HUG’s strong points is their clinical research. Through close and active collaboration with the World Health Organisations (WHO), HUG reaches a world-wide scientific audience, enjoying strong recognition both nationally and internationally.
Florence Chiodini, PhD, is a biologist at HUG’s Ophthalmology Clinic. She is in charge of coordinating the Geneva Cornea Bank’s activities.
The Geneva Cornea Bank (BDCG) is a part of HUG’s Cell Therapy Laboratory. At macro level, it provides a service regulated by the Federal Office of Public Health (FOPH) which is part of the Federal Department of Home Affairs.
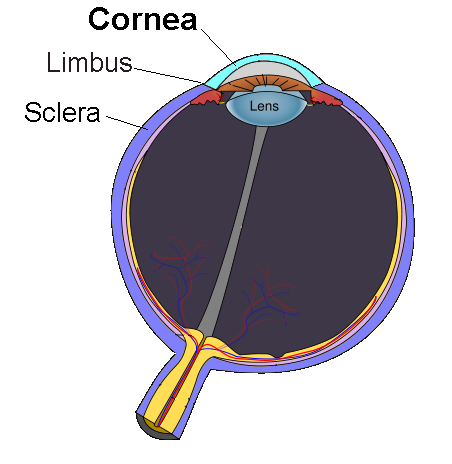
But before going any further, the image here below lets us situate our eye’s cornea.
We’re a laboratory that analyses, preserves and distributes corneas. I am in charge of coordinating the Geneva Cornea Bank’s activities.
Running a Cornea Bank is a highly regulated and controlled affair. When a person dies at HUG, clearly established processes come into play.
An important task that is assigned to me is the maintenance and management of up-to-date databases.
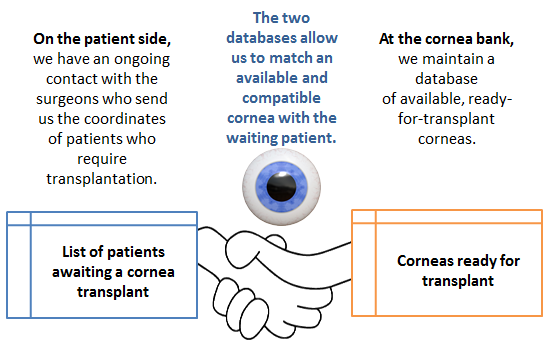
There are three demanding steps a cornea has to overcome before it may enter into the Geneva Cornea Bank.
The person responsible for the cornea bank communicates with the local donor coordinators who do the identification of potential cornea donors.
When a person has died, they consult the medical record.
During one year, there are between 1200 and 1500 deaths at the Geneva University Hospital.
When no contra-indications are found to cornea donation, the coordinator contacts the relatives to seek consent for cornea donation.
This standard procedure is always performed, even if the deceased person already gave his agreement on a donor card.
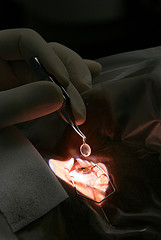
When consent is given and no other medical contra-indications are found, the coordinator organises the procurement of the corneas by an ophthalmologist.
The corneas are placed in separate sterile receptacles, containing a biological solution which ensures their conservation. 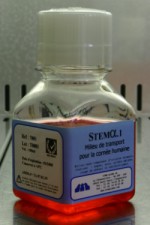
They are then deposited at the cornea bank by the coordinator and stocked in a special chamber for a period of quarantine.
The biologist is responsible for their reception and storage until serological, bacteriological and morphological analyses are complete.

.
Corneal transplantation, also known as corneal grafting or Keratoplasty is one of the most performed transplantation operations in the world. Over 100’000 interventions happen every year. In Switzerland, the number is around 500.
 However, we have a cornea shortage in our country, with an estimation of 800 patients awaiting a cornea transplant.
However, we have a cornea shortage in our country, with an estimation of 800 patients awaiting a cornea transplant.
Cornea banks can also be found in Bern, Zurich, Lucerne, Olten and Lausanne. In Geneva, the number of patients awaiting a cornea is estimated to be around 80.
Corneas spend only a short time at the cornea bank, 30 days being the maximum. Once admitted and ready, our challenge is to collaborate with the ophthalmologic surgeons, set priorities and get the precious tissue to the patient who needs it most.
NB: Did you know that corneal transplantations are being practiced since 1887? The physician who performed the first operation was Dr. Arthur von Hippel (1841-1916).
Rules and regulations surrounding all activities of a cornea bank are plentiful and require our utmost attention.
Geneva is a new cornea bank. We came into operation in March 2013 and we apply the most up-to-date scientific knowledge and best practice to both the bank’s infrastructure and related processes. For example, we work in a Clean Room, see next chapter.
All our processes have been certified by Swissmedic, the Swiss agency for the authorisation and supervision of therapeutic products.
Geneva Cornea Bank (BDCG) meets the highest health safety regulations, set by both Swiss and European authorities.
| Preparation & Storage | Quality Control | Release of the corneal transplant to the surgeons |
|
|
|
|
Images provided by HUG Communications Department.
Our laboratory in Geneva is the only one in Switzerland to dispose of such a space.
A Clean Room, in French “Salle Blanche”, is a well-defined area where key parameters such as temperature, humidity and pressure are constantly maintained at pre-determined levels – hence preventing environmental contamination.
This will no doubt change in future, as regulations evolve and the availability of a Clean Room becomes mandatory.
At present, our certified Clean Room reinforces Geneva University Hospital’s (HUG) reputation for excellence, and gives us a powerful strategic advantage.
As you’ve seen in the previous passages, the field we operate in is delicate and highly regulated. An absolute must is certification by Swissmedic, Switzerland’s regulation body.
We could not possibly implement the best of all processes internally (the procedures that define how corneas enter our Cornea Bank) and then fail when we release and distribute (transport) the tissue to its final destination.
We must work with Swissmedic predefined procedures and regulations at EVERY STEP of the process.
NVLogistics, and more specifically Vital Logistics, their biological and pharmaceutical transport division – are currently the only independent transporter in the French part of Switzerland to be Swissmedic certified.
This key factor, combined with their excellent reputation, made it easy for us to select NVLogistics as our preferred shipper and partner for the Cornea Bank project.
As previously stated, we operate with full certification at all levels of our supply chain and as such, we only collaborate with organisations who are Swissmedic certified.
A transporter such as certified NV Logistics is able to provide us with all the authorizations necessary – Swiss and European – to transport our corneas within Switzerland and Europe.
The transporter with whom we partner must be able to implement existing protocols & regulations regarding temperature, time, traceability and specific packaging.
NV Logistic’s advice has been precious. Our many open and stimulating discussions culminated in a customised packaging solution which fulfils our very specific needs.
Corneas are immersed in a biological solution which ensures their conservation. Maintaining the solution at pre-defined and constant temperate levels is essential for keeping the tissue at its optimum state.
We achieve this with a Triple Layer System for packaging and a fleet of vehicles which are equipped to maintain temperatures at requested levels.
The photo here below shows the various components:

Since going operational in March 2013, the majority of cornea shipments occurs on Swiss territory.
As far as Europe, we maintain close collaboration with the Tissue & Cell Bank of the Lyon Civil Hospices – Les Hospices civiles de Lyon (HCL) – with whom we’ve signed an Interbank Convention.
In case of an emergency, we can exchange matching corneas. Having a transporter who can handle international transport immediately and with all necessary certification is obviously a great advantage for all concerned.
The HUG Press Release (in French) announcing the opening of the Geneva Cornea Bank (April 2013).
.
This interview was conducted by Piero Zappaterra while a student at HES So.
Piero is candidate for an MscBA in Orientation Management and Service Engineering.
Images under Creative Commons Licence:
The schematic diagram of the human eye with thanks to Mikael Häggström
The Cornea Transplant image was generously provided by Obis International (via Flickr)
Office picture (via Kinnarps)
Our client, Debiopharm Group, transforms promising molecules into innovative therapies.

Founded in 1979 and headquartered in Lausanne, Switzerland – Debiopharm’s main area of expertise is oncology, but they also develop medication in therapeutic areas such as immunology and infectious diseases.
For your easy reading, we have divided this report into three parts.
1. The Client’s Challenge |
|
2. NV Logistics’ Answer |
|
3. The End Result |
empty
.
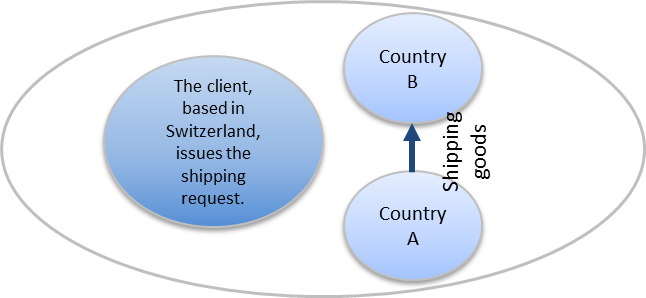
Cross trade shipping is the transportation of goods between two countries, outside of the country where the client (the paying party) is located.
Isothermal packaging, also referred to as insulated shipping systems, are solutions that conserve goods at a particular temperature for a specific period of time.
A competent shipper will dedicate his expertise to choosing the optimal system, based on a client’s exact specifications.
To learn more about packaging, please read our How to get cold chain shipping right tutorial. Your bonus: an easy to use Shipping Profile Checklist (infographic), ready for download and print.
As the handling and transport of temperature sensitive substances is becoming more tightly regulated worldwide, cold chain is a highly sought-after service. Typical users are in the life sciences field: biomedical companies, hospitals, research laboratories, and also in the food industry.
A cold chain is a system which is made up of:
Its aim is to keep substances at a guaranteed temperature range whilst transporting them from destination A to destination B.
Cold chain systems can be very complex, requiring impeccable planning and disciplined implementation by experienced shippers.
Recommended!
Check out our Cold Chain Tutorials series which include practical infographics for download and print. We provide ready to use check lists covering must-have data such as temperature, product volume, transit times, and more.
How to get Cold Chain Shipping right | Setting the scene for best value (1/3)
How to get Cold Chain Shipping right | Active and Passive shipping solutions (2/3)
.
.
NV Logistics is experienced and fully equipped to execute cross trade shipping.
Internal expertise is backed up by collaboration with worldwide partners, judiciously chosen for their know-how in the biopharmaceutical/life sciences environment. Amongst others: World Cargo Alliance (WCA) and SFS Pharma Logistics.
When a customer places a cross trade shipping order with us, we coordinate (on behalf of the client) the shipment from A-Z. Each step is documented and communicated to the client’s internal project manager.
For this specific project, goods needed to be moved from the departure location in Austria to Vienna airport. We interacted with and monitored activities with both the local packaging supplier and the local shipping representative.
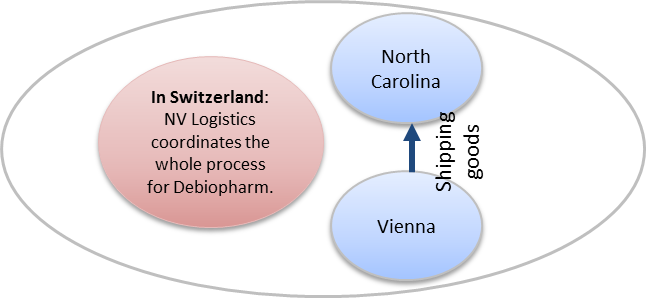
Once arrived in the US, our local shipping agent welcomed the goods at the airport, monitored the import formalities and moved the goods from the airport storage to the final client destination.
The Debiopharm shipping brief required a guaranteed and validated temperature range of between 2-8º C. In other words a non-interrupted cold chain between Austria and the USA .

Based on the client’s described needs, we evaluated several solutions and submitted our preferred choice – fully documented – to Debiopharm for their approval.
For this specific shipping request we retained:
©Laminar Medica Insulated shipping systems.
Laminar Chilltherm® CTC 506 Summer*
*The destination point being North Carolina, the average temperature range of that location at that specific season made us choose the Summer configuration.
Want to learn more about Laminar system assembly? View this video – and/or download this PDF.
NV Logistics’ internal decision processes are based on experience, technical know-how and predefined checklists. Download our temperature checklist, for your immediate practical use.
We strive to provide the safest solution at optimised cost/performance ratios.
The success of cold chain transport relies on eliminating all possible leak points throughout the whole shipping journey. When we coordinate shipping missions on behalf of our clients, these 3 elements are essential, and dear to us:
1. Safe isothermic packaging.
Each isothermal packaging system comes with its own (sometimes complex) instruction set. The availability of competent packaging staff is, therefore, essential.
For Debiopharm we ensured that:
2. Exact shipping documentation.
For optimal cold chain shipping, time is of the essence. We ensured that documents such as bills of lading and pro-forma invoices strictly conformed with the regulations of the importing country.
We have an attitude to shipping documents that could be qualified as a little pedantic 😉 but our zeal is usually rewarded! We consistently achieve highly performant import events.
For Debiopharm, we submitted initial drafts to our importing agent in the USA who suggested a few minor changes, thus ensuring smooth customs clearance.
 Debiopharm’s internal staff duly reissued revised documents which we forwarded to our local agent (USA) for final approval. We then received the “OK to ship”.
Debiopharm’s internal staff duly reissued revised documents which we forwarded to our local agent (USA) for final approval. We then received the “OK to ship”.
3. Product temperature integrity proof.
An essential component of cold chain transport is the monitoring of temperature levels throughout the journey. Monitored data in the form of temperature charts is being delivered to the client at termination.
But if the client so desires – we’re fully equipped to provide live monitoring during the journey.
For Debiopharm, we ensured that the local representative at departure point in Austria embedded adequate temperature logger devices.
Our agent in the USA arranged for the recuperation of the temperature logger devices. He made sure that the data logger reports were ready as soon as the shipment was delivered to the client’s location.
Hence the client had immediate access to crucial cold chain data which, in the end, determines the shipment’s success.
![]()
.
This is the concluding message (after delivery was completed) we received from Debiopharm’s project manager.
Thank you very much for your message and your exceptional service concerning this shipment.
Moral:
For cold chain shipping to be successful, all components must be “just right”, and each individual professional who is part of the chain needs to do “the right thing”.
We like to compare ourselves to a conductor of an orchestra. A symphony touches our heart if each individual artist is given the chance to shine – so that each single action becomes an exceptional part of an exceptional outcome.
At NV Logistics we take the trust our clients bestow on us very seriously.
One of the best ways to earn trust is complete transparency. This starts with ongoing and clear communication during the whole shipping journey.
Here are 2 examples of how we communicate.
1. Photo sent to Debiopharm’s project manager in Lausanne.

2. Client Response.
“Thank you very much for this reassuring update. I will also take advantage of your nice photos to provide the recipient with an idea of the volume this package represents”.
2. Arrival announcement sent to goods’ recipient in North Carolina (USA).
NV Logistics’ US representative sent this message to the recipient of the goods:
“Just wanted to confirm that the shipment is in good order and in route to you. With the rain and storms that have been in the area they are anticipating arrival between 13h30 and 14h30 this afternoon.
The driver will be awaiting permission to open the container and remove the two data loggers. He will take these with him.”
3. The following report, rendered by the two data loggers, was subsequently sent to the client.
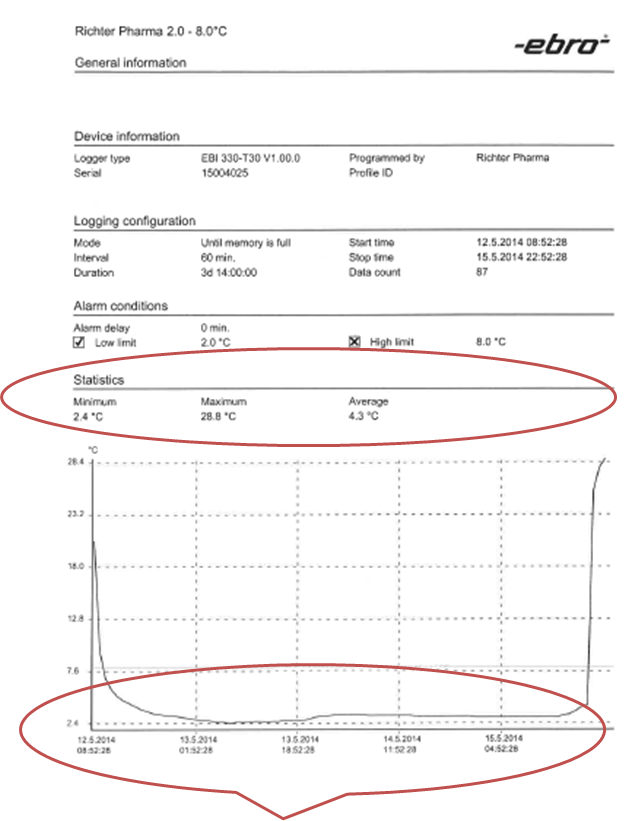
The recorded temperature data shows a consistent maintaining of the range at the requested levels, i.e. an average of 4.3° C
Photo credits:
Feature photo Vienna (Austria)
kindly provided by Flickr and photographer Martin.
Feature photo Charlotte (North Carolina)
kindly provided by Flickr and photographer James Willamor
Creative Commons License.
NV Logistics
Bâtiment fret cargo – Porte 1
Etage Mezzanine – Bureau 014
Case postale 1117
1211 Genève Aéroport
Switzerland
Tel.+41.22.566.40.00
